Abstract
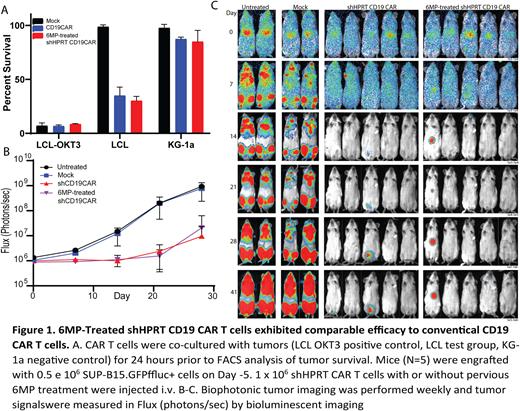
Antimetabolites, such as 6-mercaptopurine (6MP), have been a corner stone treatment of childhood acute lymphoblastic leukemia (cALL) for decades. Following induction of remission, 6MP is used in combination with other drugs as maintenance therapy to prevent relapse. Recently Gabelli et al showed treating patients with maintenance therapy, including 6MP following CD19 CAR T cells therapy had similar efficacy to patients that were treated with stem cell transplant following CD19 CAR therapy. Although encouraging, maintenance therapies, such as 6MP, are known to be toxic to lymphocytes. To address the toxicity of 6MP to CAR, we developed an anti-CD19 CAR construct that expresses a short hairpin RNA against hypoxanthine phosphoribosyltransferase 1 (HPRT), the protein the processes 6MP into its toxic form. We observed the shHRPT RNA, expressed under the U6 promoter, is sufficient to suppress HPRT expression in T cells. We observed conventional and HPRT suppressed CAR had similar phenotype and functionality in vitro and in vivo (Figure 1A-C). The suppression of HPRT is sufficient make CAR T cells resistant to 6MP and its analogue 6-thioguanine (6TG). Finally following enrichment of shHPRT-expressing CAR T cells with 6MP in vitro, the shHPRT-expressing CAR T cells also had similar antitumor activity in NSG CD19 tumormodel as those untreated with 6MP.
Overall, our studies in both in vitro and in vivo treatment support that suppressing HPRT made CAR T cells resistant to 6MP. We believe by making CAR T cells resistant to present treatments, we can increase the remission rates. Our data provides rationale to develop novel CARs resistant to well established chemo-agents to potentially aid in CAR T cell therapy for both hematological and solid tumors.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal